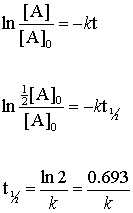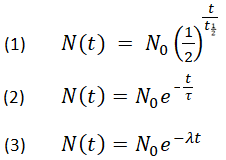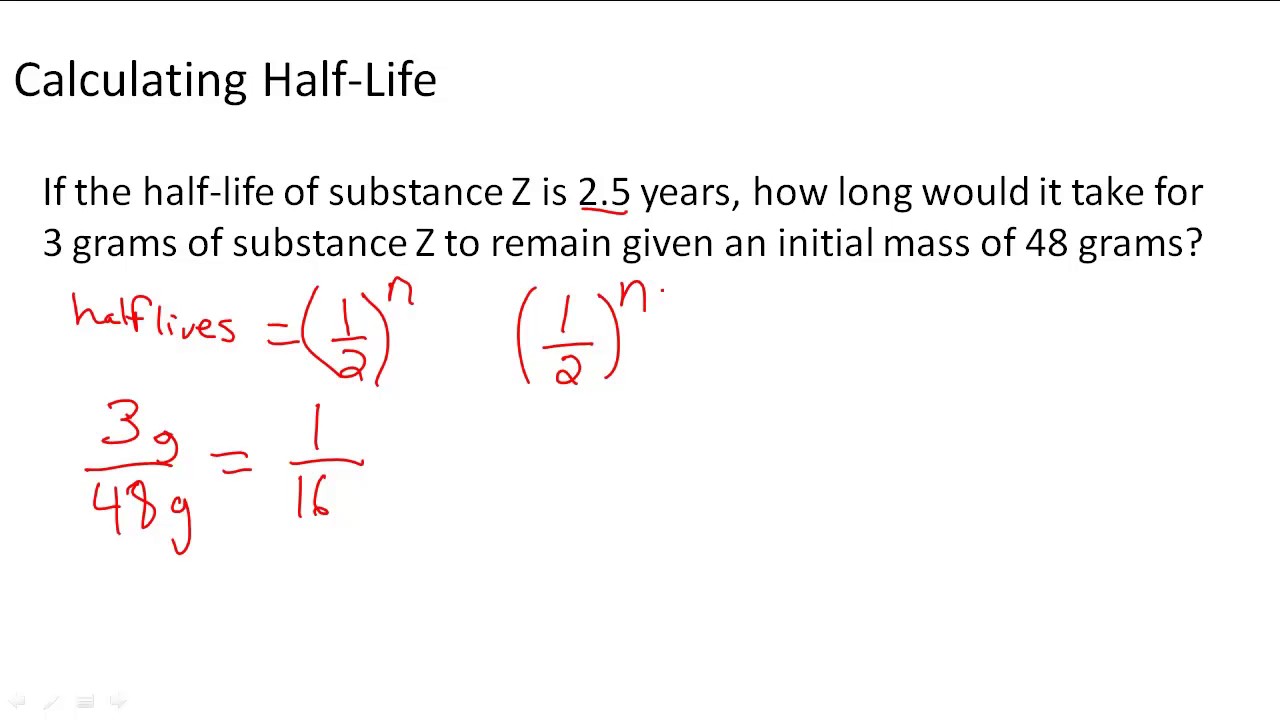half life formula chemistry
Calculate the half-life of a radioactive substance whose disintegration constant happens to be 0002 per year. For 1st order reaction k 0693t12 0693.

Half Life And Radioactive Decay Rates Online Chemistry Tutorials
The half-life for the radioactive decay of 14C is 5730 years.

. Nt No e -λ t12 Why do we calculate half-life. The half-life formula used to calculate zero order reaction is t₁₂ A₀2k. For example if the half-life of a 500 gram sample is 3 years then in 3 years only 25 grams.
T ½ 1 k A o Top Determining a Half Life To determine a half life t ½ the time required for the initial concentration of a reactant to be reduced to one-half its initial value we need to know. What is the formula for calculating half-life. Half-life formula and unit for first.
Hence the half-life of this particular. N t N 0 05 t T In which N 0 is the number of atoms you start with and N t the number of atoms left after a certain time t for a. T_ 12 frac 1 A_0k.
The formula for the half-life is obtained by dividing 0693 by the constant λ. Here λ is called the disintegration or decay constant. The general equation with half life.
Assuming first-order kinetics see Section 12 the equation for the half-life is as follows. Half Life Chemistry Problems - Nuclear Radioactive Decay Calculations Practice Examples 915095 views Sep 22 2016 This chemistry video tutorial shows explains how to. The unit of half-life equation for zero order reaction is second 2.
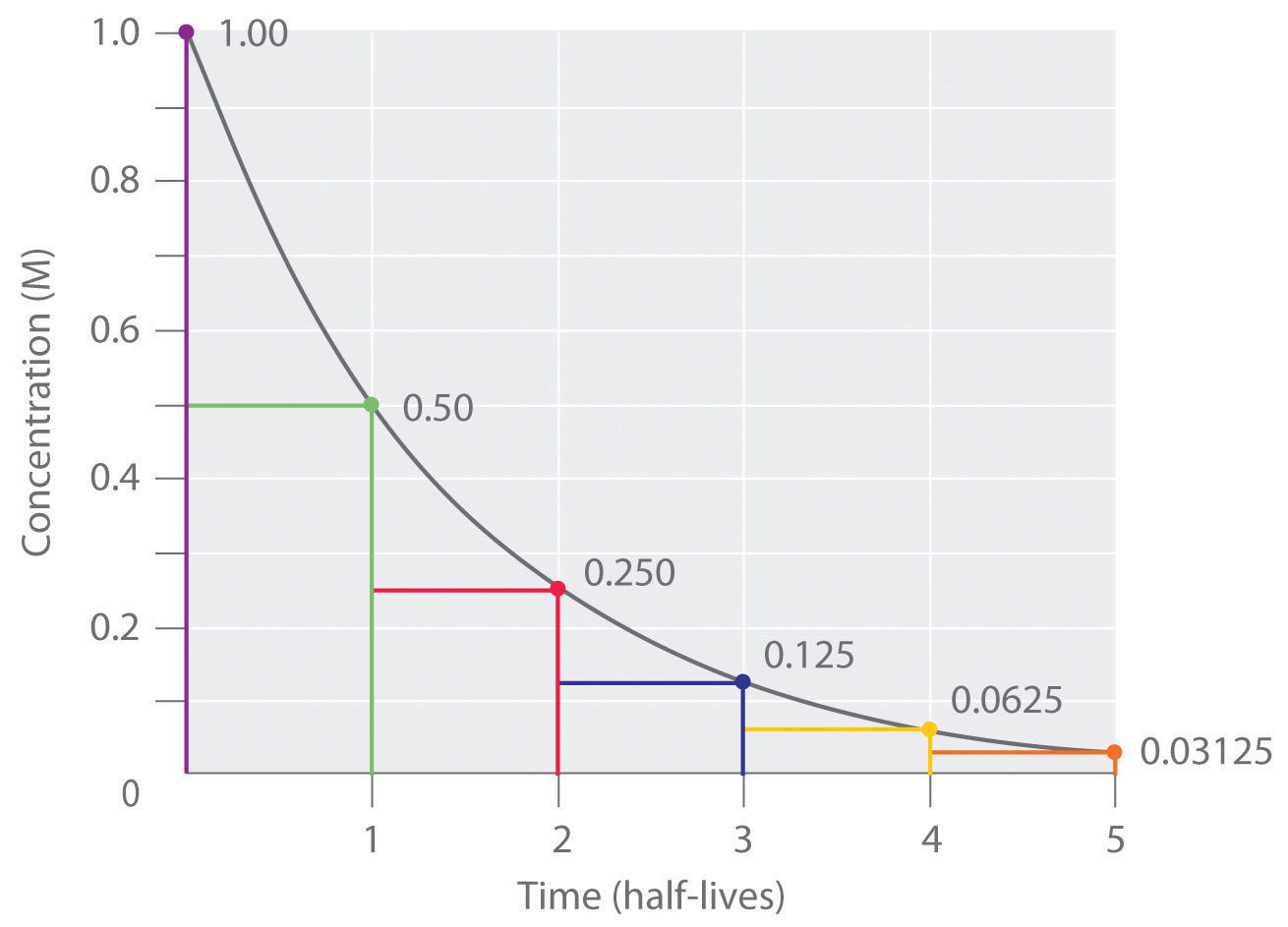
Hence the formula to calculate the half-life of a substance is. Half-life Half-life thalf is defined as the amount of time required for the amount of a substance to be reduced by 50. Half-life symbol t 12 is the time required for a quantity to reduce to half of its initial valueThe term is commonly used in nuclear physics to describe how quickly unstable atoms undergo.
An archaeological artifact containing wood had only 80 of the 14C found in a living tree. Using the half-life equation derived from the concentration-time equation as shown in example 1 we can solve for the initial concentration of reactant. Half-life a useful concept if its value does not depend on how much.
Half-life can be calculated by the given formula. Half-Life t ½ the time required for the number of nuclides to reach half the original value. Estimate the age of the sample.
The half-life has importance for the quantification. Half life formula of nth order reaction is t 1 2 2 n 1 1 A 0 n 1 n 1 k Problem practice If the half life period for a first order reaction in A is 2 minutes How long will it take A to reach 25 of its initial concentration. Solved Examples for Half Life Formula.
A radioactive half-life refers to the amount of time it takes for half of the original isotope to decay.

Elimination Half Life An Overview Sciencedirect Topics
Half Lives And Radioactive Decay Kinetics

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
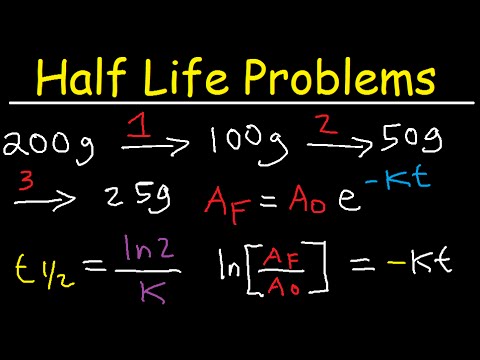
Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube

5 Ways To Calculate Half Life Wikihow
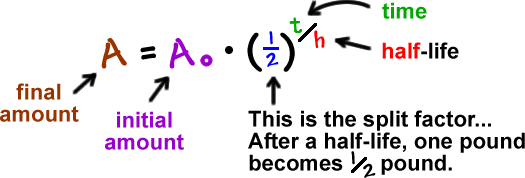
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels
Radioactive Decay Equation Formula Nuclear Power Com
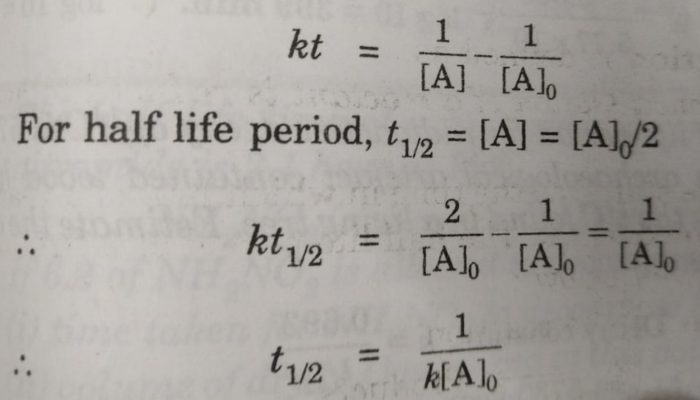
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
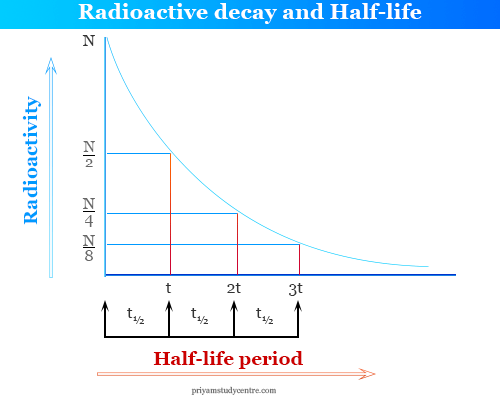
Radioactive Decay Half Life Definition Formula Calculation

How To Find Half Life Algebra Study Com

Ap Ab Calculus Half Lives Objective To Derive The Half Life Equation Using Calculus To Learn How To Solve Half Life Problems To Solve Basic And Challenging Ppt Download

Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube
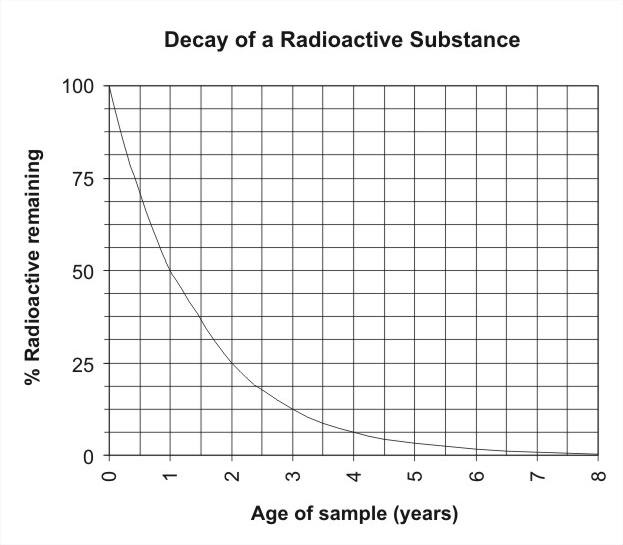
Relationship Between Radioactive Decay And Half Life Definition Mechanisms Examples